Introduction
Toxoplasmosis is a protozoan disease caused by Toxoplasma gondii, the zoonotic parasite belonging to the phylum Apicomplexa. Members of the Felidae family are the definitive hosts of T. gondii. Any warm-blooded vertebrate can act as the intermediate host through ingestion of tissue cysts in other hosts or sporulated oocysts in contaminated environments [Reference Tenter, Heckeroth and Weiss1]. Humans can be infected by ingesting T. gondii tissue cysts in undercooked meat of infected animals or oocysts in environments contaminated by the faeces of primarily infected cats. Once ingested, tissue cysts are formed in human muscle or brain tissue, and latent infection is established [Reference Montoya and Liesenfeld2]. One-third of the world’s population is generally assumed to have been infected with T. gondii. Infections are mostly asymptomatic and/or self-limited; however, severe consequences are more common in the developing foetus and immunocompromised individuals [Reference Tenter, Heckeroth and Weiss1, Reference Montoya and Liesenfeld2].
Vertical transmission to the foetus via the placenta can occur in women with primary T. gondii infection during pregnancy or just before pregnancy and can lead to spontaneous abortion or congenital toxoplasmosis (CT) in some infants. Two meta-analyses on the risk of primary infection among pregnant people consistently reported that acute T. gondii infection in pregnant people, primarily determined by specific IgM detection or several serological criteria combined, is 1.9% and 1.1% worldwide, respectively [Reference Bigna3, Reference Rostami4]. Globally, the annual CT number is estimated to be 190100 cases (95% confidence interval [CI]: 179300–206300), accounting for 1.2 million disability-adjusted life years (DALYs) annually (one DALY is equivalent to one year of healthy life lost) [Reference Torgerson and Mastroiacovo5]. Pyrimethamine jointly used with sulfadiazine is approved for treatment of CT in several countries [6, 7]. Spiramycin is the only available medication to prevent vertical transmission of T. gondii among pregnant people with primary infection [Reference Montoya and Liesenfeld2, 8].
The reported seroprevalence of T. gondii in the general Japanese population is 9.3%–16.4% [Reference Abdelbaset, Abushahba and Igarashi9–Reference Yamaoka11]. Sakikawa et al. [Reference Sakikawa12] reported a seroprevalence of 10.3% among pregnant people who received antenatal care in a private hospital during 1997–2004. Other studies in Japanese language have documented a seroprevalence from 2% to 10% among pregnant people [8, Reference Nishikawa13–16]. The risk of T. gondii during pregnancy has not been well studied. Sakikawa et al. [Reference Sakikawa12] reported a 0.25% acute infection rate during pregnancy. A guideline for T. gondii screening and treatment for primary infection during pregnancy is available in Japan [8]. According to the guideline, when serum T. gondii IgG is positive in the first trimester, T. gondii IgM is measured. The T. gondii IgG avidity index may also be considered, although this is not mandatory. If T. gondii IgM is positive, primary T. gondii infection during pregnancy is suspected, and prompt therapy with spiramycin is strongly recommended. Amniocentesis may be performed based on the pregnant woman’s preference. If foetal infection is confirmed by detection of T. gondii DNA, maternal therapy with pyrimethamine, sulfadiazine, and folinic acid is administered between 16 and 27 weeks of gestation. Because pyrimethamine and sulfadiazine are not approved in Japan, their use is restricted to designated medical institutions. From 28 weeks of gestation, sulfadiazine is discontinued and pyrimethamine and folinic acid (or spiramycin) are given until delivery. If amniocentesis is not done or if foetal infection is not confirmed by polymerase chain reaction (PCR), spiramycin is given until delivery. For primary infections detected later in pregnancy, the same management with cases detected earlier is applied. Notification of CT is not mandated in Japan, and thus, it is unclear how many cases are present at a national level in Japan. Yamada et al. [Reference Yamada17] reported the rate of CT at 1.5% (7/469) in Japanese women testing positive for T. gondii between April 2005 and July 2017. However, in a prospective study at two university hospitals from 2013 to 2020, Hijikata et al. [Reference Hijikata18] reported that among 73 newborns from 71 T. gondii IgM-positive mothers, none had clinical manifestations and T. gondii-specific IgM antibodies were detected in one (approximately 70% of participants had preventive therapy with acetylspiramycin or spiramycin). To address the number of CT cases at national level using a commercial claims database covering 17 million employed persons and their relatives, the yearly number of CT cases from 2010 to 2018 was recently estimated to be 295, corresponding to 2.9 cases per 10000 births [19]. In studies using International Classification of Diseases Tenth Revision (ICD-10) codes in claims databases, the validity of coding can be a limitation. Moreover, the generalizability of findings based only on employed workers relative to the entire Japanese population should be considered. The epidemiology of toxoplasmosis among pregnant people and CT in Japan remains to be quantitatively elucidated.
The National Database (NDB) is a Japanese database that stores and organises data such as medical receipt information and specific health checkup and health guidance information [20]. The NDB is an open data source on selected reimbursed health care services during particular fiscal years, including prescribed medications in all prefectures [21]. Spiramycin was approved for the prevention of CT in Japan in 2018 and it is the only approved drug for this indication. On the other hand, spiramycin is used only for the prevention of CT, and not for the treatment of other infections. The use of a mathematical modelling approach with local serological data, combined with the rate of detection of primary T. gondii infection during pregnancy, enables estimating the burden of disease caused by the parasite, including the number of primary T. gondii infections in pregnant people and CT cases. In the present study, we estimated the T. gondii infection risk during pregnancy using a compartment model capturing the infection dynamics in women during pregnancy, further assessing primary infections among pregnant people and vertical transmission of T. gondii in Japan.
Methods
Data collection strategy for estimation
The prescribed number of spiramycin doses is recorded, encompassing all treatment courses received by pregnant people following a first-ever diagnosis of T. gondii infection during pregnancy. We assumed that the risk of pregnant people acquiring the infection depends on the prefecture of residence (characterising the exposure); ascertainment of infection is also dependent on the local testing rate, such that the fraction of infected pregnant people receiving treatment with spiramycin also differs by prefecture. To describe the process of estimating the T. gondii infection rate based on spiramycin prescriptions, we used the datasets described below.
Number of new pregnancies
Data on pregnancies in Japan are reported on a yearly, not monthly, basis, except for specific periods shown later [22]. Rather than using these data, we leveraged the monthly reported number of births in each prefecture [23]. Assuming all reported births had a gestation period of 10 months, we used the number of births in (X + 9) calendar month as the number of new pregnancies in X calendar month. For the period January 2018 to October 2021, the number of pregnancies was mandated to be reported each month to investigate the impact of COVID-19 on pregnancy [24]. Most pregnancies (i.e., >90%) were reported by 11 weeks of gestation [24]. Thus, for this specific period, we used the monthly reported number of pregnancies in (X + 2) calendar month as the number of new pregnancies in X calendar month.
Seroprevalence of T. Gondii among pregnant people
Data on the seroprevalence of T. gondii among pregnant people, indicating previous T. gondii infection, are available for selected prefectures in Japan, i.e., Hokkaido (3.6%), Saitama (3.3%), Chiba (4%), Tokyo (6%), Osaka (6%), Hyogo (3.5%), Yamaguchi (5.3%), Nagasaki (2.1%), and Miyazaki (10%) [8, Reference Sakikawa12–16, 25]. Additionally, a nationwide multi-centre study by the Ministry of Health, Labour and Welfare of Japan reported that the seroprevalence at the national level during 2013–2015 was 6.1% [8]. Once infected with T. gondii, the host generally remains infected for life and develops life-long immunity against reinfection; therefore, only primary infection was taken into account and no reinfections were assumed in the model. We targeted the above prefectures in the following analysis, using the value (1 − seroprevalence) as the proportion of susceptible pregnant people.
Testing rate of T. Gondii in selected prefectures
Biennial national-level surveys targeting all hospitals offering obstetrics or gynaecology services in Japan are used to report the proportion of hospitals that routinely check for acute T. gondii infection during pregnancy in each prefecture [26]. The average proportion of testing in 2019 and 2021 was retrieved for our analysis. In sensitivity analyses to determine the impact of the testing rate on our estimate of the risk of infection, we calculated 95% CI of the proportion in both years, assuming a binominal distribution (Supplementary Table S1). The lower bound or upper bound of the 95% CI was used as the pessimistic or optimistic scenario of the testing conditions, respectively.
Spiramycin prescription in Japan
The prescribed number of spiramycin doses per fiscal year from 2018 (i.e., April 2018 to March 2019) to 2021 is available across Japan [21]. Because spiramycin treatment was initially set up in practice from August 2018 [8], and the adoption and procurement of newly approved medications require some time, the data for fiscal year 2018 were excluded in the following analysis.
Transmission model of T. Gondii infection in pregnant people
We used a simple catalytic susceptible–infected (SI) model to describe the transmission dynamics of T. gondii among pregnant people over the course of time and by age [Reference Vynnycky and White27]. Letting
![]() $ {S}_i(t) $
be the time-dependent number of susceptible pregnant people at calendar time
$ {S}_i(t) $
be the time-dependent number of susceptible pregnant people at calendar time
![]() $ t $
and in prefecture
$ t $
and in prefecture
![]() $ i $
, the incidence of infection among pregnant people in gestational month
$ i $
, the incidence of infection among pregnant people in gestational month
![]() $ a $
at calendar time
$ a $
at calendar time
![]() $ t $
and in prefecture
$ t $
and in prefecture
![]() $ i $
is described as
$ i $
is described as
for a = {0, …, 9}, where
![]() $ {\lambda}_i $
is the risk of endemic infection with T. gondii in prefecture
$ {\lambda}_i $
is the risk of endemic infection with T. gondii in prefecture
![]() $ i $
. Notably, infections estimated to occur one month before pregnancy (gestation month zero) were included among infections during pregnancy because 12 weeks are required for IgG to reach the peak value after infection [Reference Fricker-Hidalgo28], and infections acquired in the month prior to pregnancy will be detected in early pregnancy. Assuming that the number of women at calendar time
$ i $
. Notably, infections estimated to occur one month before pregnancy (gestation month zero) were included among infections during pregnancy because 12 weeks are required for IgG to reach the peak value after infection [Reference Fricker-Hidalgo28], and infections acquired in the month prior to pregnancy will be detected in early pregnancy. Assuming that the number of women at calendar time
![]() $ t-1 $
who will eventually become pregnant at calendar time
$ t-1 $
who will eventually become pregnant at calendar time
![]() $ t $
is equal to the number of pregnant people at gestational month zero at calendar time
$ t $
is equal to the number of pregnant people at gestational month zero at calendar time
![]() $ t+1 $
, we modelled infections 1 month prior to the pregnancy (i.e., a = −1) at calendar time
$ t+1 $
, we modelled infections 1 month prior to the pregnancy (i.e., a = −1) at calendar time
![]() $ t $
and in prefecture
$ t $
and in prefecture
![]() $ i $
as
$ i $
as
![]() $ {S}_i\left(t+1\right)\left(1-\exp \left(-{\lambda}_i\right)\right) $
.
$ {S}_i\left(t+1\right)\left(1-\exp \left(-{\lambda}_i\right)\right) $
.
Modelling the number of infected women starting spiramycin
A nationwide survey of screening practice during pregnancy shows that the testing for T. gondii infection takes place only once during the course of pregnancy (789/803 hospitals, 98%) [Reference Yamada29]. We let g(
![]() $ \sigma $
|
$ \sigma $
|
![]() $ a $
) be the probability density function of the time delay from infection to testing
$ a $
) be the probability density function of the time delay from infection to testing
![]() $ \sigma $
, conditional on gestational month
$ \sigma $
, conditional on gestational month
![]() $ a $
of infection. Assuming that all infections, if tested for, would be judged as confirmed/suspected infection, and spiramycin is started in the month of testing, the number of infected women starting spiramycin in calendar month
$ a $
of infection. Assuming that all infections, if tested for, would be judged as confirmed/suspected infection, and spiramycin is started in the month of testing, the number of infected women starting spiramycin in calendar month
![]() $ t $
and in prefecture
$ t $
and in prefecture
![]() $ i $
can be described as
$ i $
can be described as
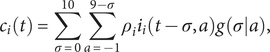 $$ {c}_i(t)=\sum_{\sigma =0}^{10}\sum_{a=-1}^{9-\sigma }{\rho}_i{i}_i\left(t-\sigma, a\right)g\left(\sigma |a\right), $$
$$ {c}_i(t)=\sum_{\sigma =0}^{10}\sum_{a=-1}^{9-\sigma }{\rho}_i{i}_i\left(t-\sigma, a\right)g\left(\sigma |a\right), $$
where
![]() $ {\rho}_i $
is the testing rate for T. gondii in prefecture
$ {\rho}_i $
is the testing rate for T. gondii in prefecture
![]() $ i $
. Considering that (1) anti- T. gondii IgM, indicative of acute infection, becomes positive 4 weeks after infection [Reference Fricker-Hidalgo28] and (2) nearly all testing occurs during the first trimester (i.e., gestational months 0–2) in Japan (752/753 hospitals) [Reference Yamada29], only pregnant people infected in gestational months −1, 0, or 1 were considered to have a chance of being diagnosed and receiving spiramycin. For a time delay from infection to testing conditional on gestational month at infection −1, 0, or 1, we adopted the mid-point of possible values, i.e., 1, 2, or 2 months, respectively, to calculate the gestational month of testing positive.
$ i $
. Considering that (1) anti- T. gondii IgM, indicative of acute infection, becomes positive 4 weeks after infection [Reference Fricker-Hidalgo28] and (2) nearly all testing occurs during the first trimester (i.e., gestational months 0–2) in Japan (752/753 hospitals) [Reference Yamada29], only pregnant people infected in gestational months −1, 0, or 1 were considered to have a chance of being diagnosed and receiving spiramycin. For a time delay from infection to testing conditional on gestational month at infection −1, 0, or 1, we adopted the mid-point of possible values, i.e., 1, 2, or 2 months, respectively, to calculate the gestational month of testing positive.
Modelling the prescribed number of spiramycin doses
The Japanese guideline on toxoplasmosis during pregnancy recommends continuing treatment with spiramycin until delivery [8]. Spiramycin is not effective for an infected foetus confirmed by PCR and treatment is usually switched to pyrimethamine and sulfadiazine for foetal prenatal treatment between 16 and 27 weeks of gestation. Pyrimethamine and sulfadiazine are available in only four university hospitals in Japan as of this writing [8], and a report from one site among these four hospitals mentions that spiramycin or acetylspiramycin, which was used before approval of spiramycin in 2018, was given between 16 and 27 weeks in their routine practice (total of 52 pregnant people received treatment from 2013 to 2020) [Reference Hijikata18]. Thus, we assumed in our model that spiramycin would be continuously administered until delivery, regardless of the amniocentesis results. Considering that the approved regimen of spiramycin is six tablets (1.5 M units/tablet) per day (such that, on average, 180 tablets are consumed per month), the total number of spiramycin doses prescribed in calendar month
![]() $ t $
and in prefecture
$ t $
and in prefecture
![]() $ i $
will be:
$ i $
will be:
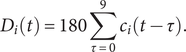 $$ {D}_i(t)=180\sum_{\tau =0}^9{c}_i\left(t-\tau \right). $$
$$ {D}_i(t)=180\sum_{\tau =0}^9{c}_i\left(t-\tau \right). $$
The total number of spiramycin doses prescribed in fiscal year
![]() $ y $
and in prefecture
$ y $
and in prefecture
![]() $ i $
can be written as
$ i $
can be written as
![]() $ {\sum}_{t\in y}{D}_i(t) $
. Assuming that infection with T. gondii among pregnant people is endemic during 2019–2021, the expected value of the total number of spiramycin doses in prefecture
$ {\sum}_{t\in y}{D}_i(t) $
. Assuming that infection with T. gondii among pregnant people is endemic during 2019–2021, the expected value of the total number of spiramycin doses in prefecture
![]() $ i $
during any fiscal year would be the arithmetic mean of
$ i $
during any fiscal year would be the arithmetic mean of
![]() $ {\sum}_{t\in y}{D}_i(t) $
for all fiscal years (i.e., identical doses for 2019, 2020, and 2021), or
$ {\sum}_{t\in y}{D}_i(t) $
for all fiscal years (i.e., identical doses for 2019, 2020, and 2021), or
![]() $ \frac{1}{3}{\sum}_y{\sum}_{t\in y}{D}_i(t) $
. Letting the observed number of prescribed spiramycin doses in fiscal year
$ \frac{1}{3}{\sum}_y{\sum}_{t\in y}{D}_i(t) $
. Letting the observed number of prescribed spiramycin doses in fiscal year
![]() $ y $
and in prefecture
$ y $
and in prefecture
![]() $ i $
follow a normal distribution, the likelihood of estimating
$ i $
follow a normal distribution, the likelihood of estimating
![]() $ {\lambda}_i $
, the risk of infection in prefecture
$ {\lambda}_i $
, the risk of infection in prefecture
![]() $ i $
, is written as:
$ i $
, is written as:
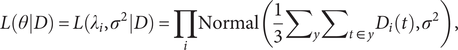 $$ L(\theta |D)=L({\lambda}_i,{\sigma}^2|D)=\prod \limits_i\mathrm{N}\mathrm{o}\mathrm{r}\mathrm{m}\mathrm{a}\mathrm{l}\left(\frac{1}{3}{\sum}_y{\sum}_{t\in y}{D}_i(t),{\sigma}^2\right), $$
$$ L(\theta |D)=L({\lambda}_i,{\sigma}^2|D)=\prod \limits_i\mathrm{N}\mathrm{o}\mathrm{r}\mathrm{m}\mathrm{a}\mathrm{l}\left(\frac{1}{3}{\sum}_y{\sum}_{t\in y}{D}_i(t),{\sigma}^2\right), $$
where
![]() $ {\sigma}^2 $
is the variance of a normal distribution, assumed to be the same across fiscal years and prefectures. As a sensitivity analysis, we assumed that a number of people infected before pregnancy will start spiramycin if they had to be managed as suspected primary infection during pregnancy because of (1) detection of specific persistent IgM antibodies persisting from an earlier infection and (2) no IgG avidity test performed to rule out past infection, to determine the robustness of estimated infection risk in the base model. Owing to limited information on the rate of IgG avidity testing in Japan, this supplementary analysis was restricted to two prefectures (i.e., Tokyo and Hyogo) (see the Supplementary text).
$ {\sigma}^2 $
is the variance of a normal distribution, assumed to be the same across fiscal years and prefectures. As a sensitivity analysis, we assumed that a number of people infected before pregnancy will start spiramycin if they had to be managed as suspected primary infection during pregnancy because of (1) detection of specific persistent IgM antibodies persisting from an earlier infection and (2) no IgG avidity test performed to rule out past infection, to determine the robustness of estimated infection risk in the base model. Owing to limited information on the rate of IgG avidity testing in Japan, this supplementary analysis was restricted to two prefectures (i.e., Tokyo and Hyogo) (see the Supplementary text).
Estimation of parameters
We used a Bayesian hierarchical model to estimate the parameters of interest. That is, the risk of infection in each prefecture was assumed to follow a gamma distribution, whose shape and rate parameters were assumed to follow the same normal distribution with mean = 0 and variance = 1. The prior variance of normal distribution for the number of spiramycin doses,
![]() $ {\sigma}^2 $
, was assumed to follow a uniform distribution bounded by zero. The Bayesian inference was implemented in RStan [Reference Gabry30]. Markov chain Monte Carlo (MCMC) with the No-U-Turn Sampler algorithm was used to estimate model parameters. The Gelman–Rubin (GR) statistic (R-hat) was used to judge MCMC convergence with a threshold of <1.1.
$ {\sigma}^2 $
, was assumed to follow a uniform distribution bounded by zero. The Bayesian inference was implemented in RStan [Reference Gabry30]. Markov chain Monte Carlo (MCMC) with the No-U-Turn Sampler algorithm was used to estimate model parameters. The Gelman–Rubin (GR) statistic (R-hat) was used to judge MCMC convergence with a threshold of <1.1.
Prediction of CT in Japan
The incidence of CT per year was predicted using estimated parameters. To predict primary infections during pregnancy, we included the estimated risk of primary infection with T. gondii into equation (1). The transmission risk when treated with spiramycin is known to vary depending on the gestation period of the pregnant woman, i.e., 10% in the first trimester, 20% in the second, and 60%–70% in the third trimester [Reference Hohlfeld31]. Because spiramycin is suggested to reduce the risk of transmission by 50% according to several observational studies [Reference Mandelbrot32], in our computations, we assumed that the risk of transmission without treatment is 20% in the first trimester, 40% in the second, and 100% in the third trimester.
All analyses were conducted using R version 4.3.1 [33]. The code is available from https://github.com/koudylan/toxoplasma_japan.
Ethical considerations
The present study used publicly available data. The datasets did not contain any individual identifying information; therefore, ethical approval was not required for this study.
Results
Infection risk of T. Gondii in Japan
The observed number of prescribed spiramycin doses in the selected prefectures is shown in Figure 1. The total number of doses prescribed nationally in fiscal years 2019, 2020, and 2021 was 252551, 353535, and 399522, respectively (Supplementary Table S2). No prescriptions were recorded in Yamaguchi Prefecture except for 2021, and thus, we excluded this prefecture from further analyses to ensure certainty in our estimates. Assuming that the risk follows a gamma distribution, the nationwide risk of infection was estimated to be 0.016% per month (95% credible interval [CrI]: 0.009–0.029) (Table 1). The risk estimates in selected prefectures ranged from 0.008% to 0.030% per month, with the largest value seen in Tokyo. The variance of normal distribution for spiramycin doses was estimated to be 0.48 (95% CrI: 0.35–0.72). The number of prescribed spiramycin doses in each fiscal year from 2019 to 2021 allowed our model to be reasonably well fitted to the observed pattern (Figure 1). The estimates of rate/shape parameters are shown in Supplementary Tables S3 and S4. Under the pessimistic scenario with a low testing rate, the risk of infection was estimated at 0.017% per month (95% CrI: 0.010–0.030) nationwide and 0.013–0.038 at the prefectural level; under the optimistic scenario with a high testing rate, the risk of infection was 0.015% per month (95% CrI: 0.009–0.026) nationwide and ranged from 0.006 to 0.025 at the local level (Table 1). When considering the proportion of people infected before pregnancy, the risks in Tokyo and Hyogo were estimated to be 0.02% per month (95% CrI: 0.00–0.07) and 0% (95% CrI: 0.00–0.02), respectively.
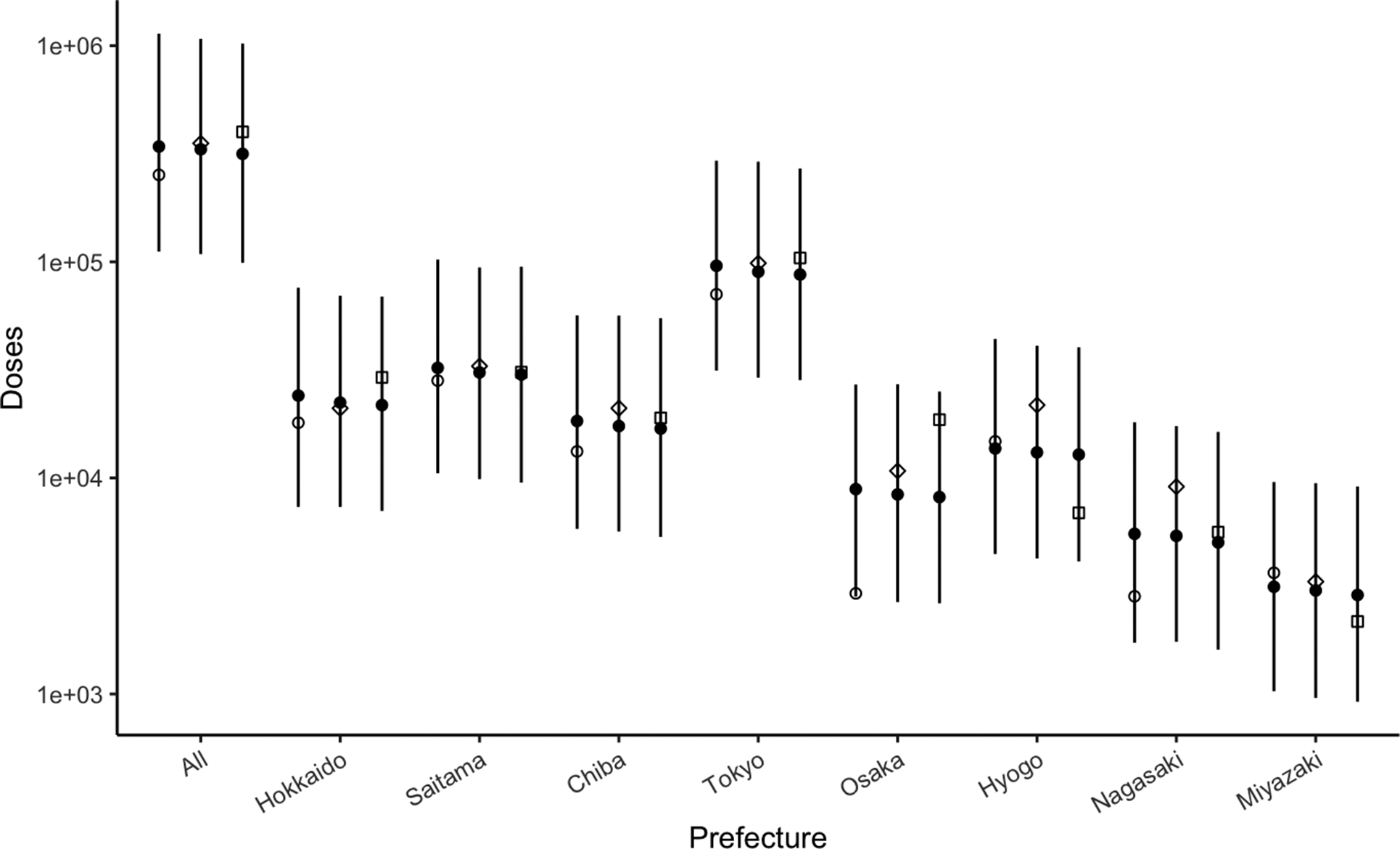
Figure 1. Prescribed spiramycin in Japan. Open circles, diamonds, and squares represent observed number of doses (in log-scale) in 2019, 2020, and 2021, respectively. Solid circles represent estimated number for the corresponding year. The line shows 95% credible interval for the corresponding year.
Table 1. Infection risk of Toxoplasma gondii during pregnancy (median and 95% credible interval)
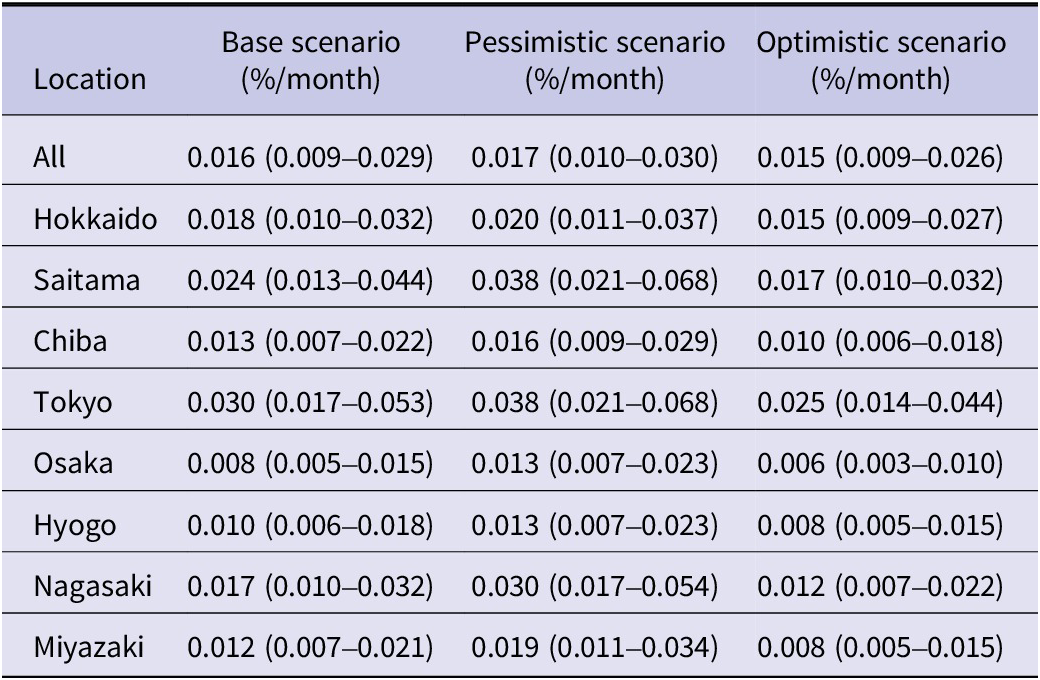
Number of T. gondii infections in pregnant people
The number of T. gondii infection among pregnant people nationwide in the years 2019, 2020, and 2021 was estimated to be 1507 (95% CrI: 851–2732), 1440 (95% CrI: 813–2611), and 1388 (95% CrI: 784–2517), respectively. Among selected prefectures, the highest number was seen in Tokyo (362 in 2019, 345 in 2020, 330 in 2021), followed by Saitama (134 in 2019, 128 in 2020, 124 in 2021), a prefecture neighbouring Tokyo. When scaled according to the population at risk in each prefecture, the incidence of T. gondii infection per 10000 pregnant people was highest in Tokyo with 32 (95% CrI: 18–55) in 2019, 32 (95% CrI: 18–56) in 2020, and 33 (95% CrI: 18–57) in 2021 (Figure 2a; Supplementary Table S5). Sensitivity analysis varying testing rates showed that the number of infections per 10000 pregnancies nationwide was 15–18 in 2019 and 16–18 in 2020/2021 (Figure 2b; Supplementary Tables S6 and S7). Under the pessimistic scenario, the incidence of infection per 10000 pregnancies in Tokyo and Saitama was similar (39 vs. 40 in 2019, 40 vs. 41 in 2020, 40 vs. 41 in 2021). Among all infected pregnant people, 13% were estimated to receive spiramycin nationwide during 2019–2021 under the base scenario. The proportion did not change substantially under the different testing scenarios (i.e., 12% and 14% for the pessimistic scenario and the optimistic scenario, respectively). In a model with people infected before pregnancy, the number of infections per 10000 pregnancies in Tokyo and Hyogo was estimated to be 17–18 and 0.01–0.02, respectively, during 2019–2021 (Supplementary Table S11).
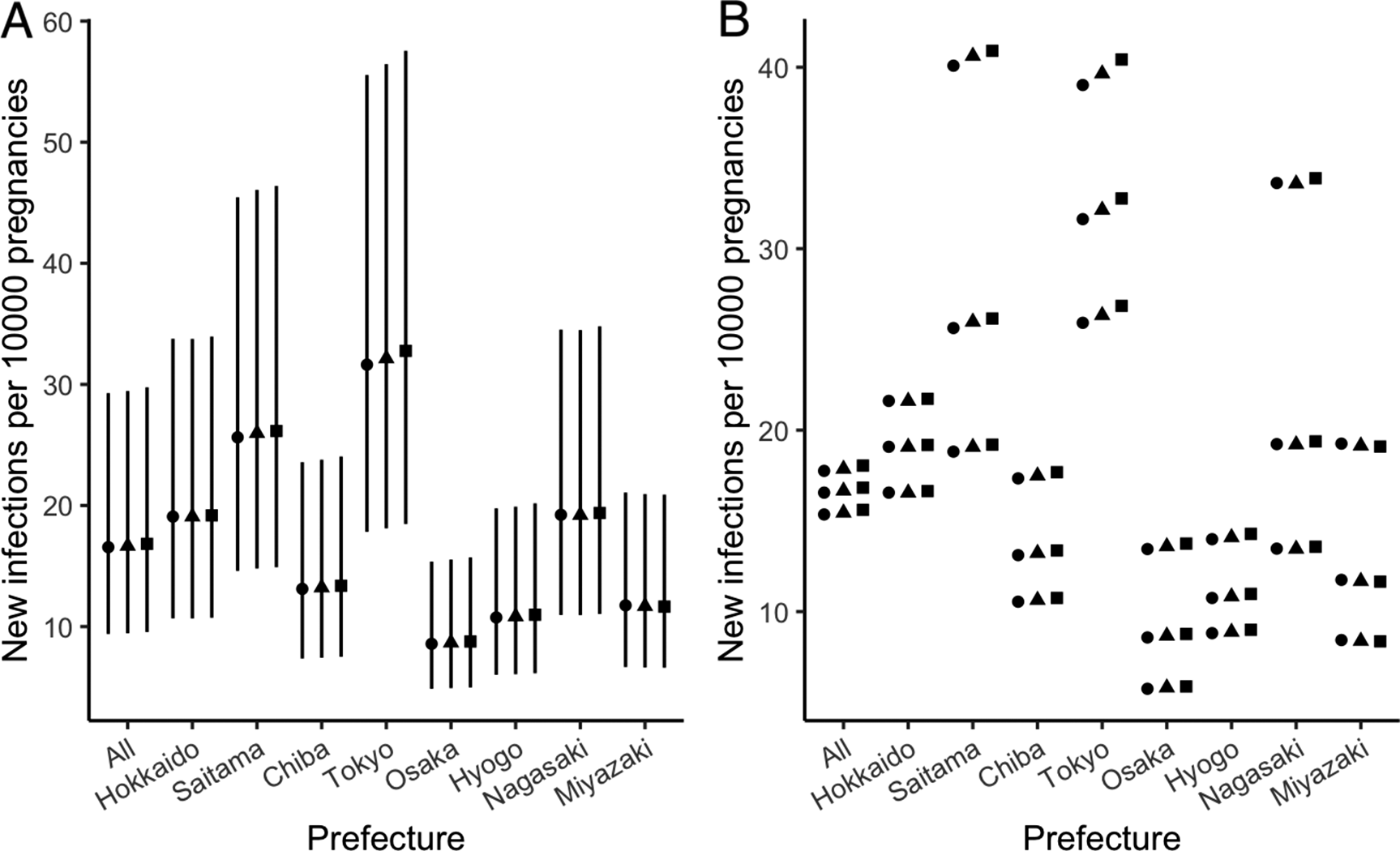
Figure 2. Incidence of Toxoplasma gondii infections during pregnancy. (a) Incidence in base scenario. Closed circles, diamonds, and squares represent estimated incidence in 2019, 2020, and 2021, respectively. The continuous black line shows 95% credible interval. (b) Incidence in base (centre), pessimistic (upper), and optimistic (lower) scenarios. Closed circles, diamonds, and squares represent estimated incidence in 2019, 2020, and 2021, respectively.
CT cases in Japan
The estimated number of CT nationwide in the years 2019, 2020, and 2021 was estimated at 613 (95% CrI: 346–1110), 588 (95% CrI: 332–1067), and 567 (95% CrI: 322–1033) cases, respectively. The largest case number was seen in Tokyo with 146 (95% CrI: 82–256) in 2019, 141 (95% CrI: 79–246) in 2020, and 135 (95% CrI: 76–236) in 2021. Figure 3a shows the incidence of CT per 10000 pregnant people in each prefecture, with the largest number in Tokyo (13 in 2019, 13 in 2020, and 13 in 2021), followed by Saitama (10 in 2019, 11 in 2020, and 11 in 2021). The incidence of CT nationwide was shown to range from 6 to 7 per 10000 pregnant people during 2019–2021, even with varying testing rates. The number of CT cases per 10000 pregnancies in Tokyo and Saitama was found to be very close under the pessimistic scenario (16 vs. 16 in 2019, 16 vs. 17 in 2020, 17 vs. 17 in 2021) (Figure 3b; Supplementary Tables S8, S9, and S10). Using risk of primary infection estimated from the sensitive analysis, the numbers of CT cases per 10000 pregnancies in Tokyo and Hyogo were estimated to be 7 and 0.01, respectively, during 2019–2021 (Supplementary Table S12).
Figure 3. Incidence of congenital toxoplasmosis. (a) Incidence in base scenario. Closed circles, diamonds, and squares represent estimated incidence in 2019, 2020, and 2021, respectively. The continuous black line shows 95% credible interval. (b) Incidence in base (centre), pessimistic (upper), and optimistic (lower) scenarios. Closed circles, diamonds, and squares represent estimated incidence in 2019, 2020, and 2021, respectively.
Discussion
We examined prescription data for spiramycin, which is uniquely and very specifically used for the prevention of CT during pregnancy in Japan, to estimate the infection risk of T. gondii nationwide and at prefectural level in selected prefectures. The nationwide risk was estimated to be 0.016% per month. The estimate was robust, even when we assumed two alternative testing scenarios (i.e., pessimistic and optimistic percentages of pregnant people undertaking testing), with estimates ranging from 0.015% to 0.017% per month. Among the prefectures investigated, the risk estimate was highest in Tokyo with 0.030% per month. Nationally, the number of T. gondii infections among pregnant people in 2019, 2020, and 2021 was estimated to be 1507, 1440, and 1388, respectively. We also estimated the nationwide number of CT in each year at 613, 588, and 567 cases, respectively. The number of CT per 10000 pregnant people at prefecture level was highest in Tokyo, estimated at 13 cases from 2019 to 2021.
To our knowledge, the present study is the first to explicitly quantify the risk of infection with T. gondii at national level as well as the incidence during pregnancy in Japan. Using a model-based approach, we predicted the number of primary infections in pregnancy, including those that could not be diagnosed and treated under the current testing rate and practice. In a modelling study focused on women of childbearing age in France, the incidence was estimated at 2.4/1000 cases in 2010 and was predicted to be 1.6/1000 in 2020 [Reference Nogareda34]. Using a healthcare claims database in Germany, the incidence of toxoplasmosis during pregnancy (defined as the first diagnosis in the study period but not in the pre-observational period) was reported to be 29.3 (95% CI: 16.7–56.4)/100000 in 2011 and 60.3 (95% CI: 33.0–107.5)/100000 in 2015 [Reference Krings35]. Our estimate of the incidence during pregnancy (16/10000 pregnancies) is comparable to the one in France and numerically higher than in Germany. Our estimated annual number of CT cases was consistently larger than that in a report based on diagnosed CT cases (with an ICD-10 code) using a claims database in Japan (295, 95% CI: 226–363) [19]. The database includes only health insurance claims for employees of large companies and does not cover other health insurance data, such as national health insurance [Reference Nagai36]. The potential biased distribution in socioeconomic status of insured people in the database may lead to underestimation of CT cases. Although the study approaches differed, both studies indicate that CT constitutes a serious burden among neonates in Japan. Considering its magnitude as compared with the reported case number for congenital syphilis (i.e., 20–30 cases annually) in Japan [Reference Hayata37, 38], greater effort is needed to ensure prevention (e.g., no exposure to undercooked meat) and proper screening of T. gondii during pregnancy.
Although the number of prefectures explored in this study was limited, our findings suggest possible spatial heterogeneities in the risk of T. gondii, at least among pregnant people, and consequently neonates. The incidence of both primary T. gondii among pregnant people and of CT was estimated to be consistently high in Tokyo and Saitama, a prefecture bordering the northern end of Tokyo. In contrast, prefectures in western and southern Japan (i.e., Osaka, Hyogo, and Miyazaki) showed a lower incidence of primary infections and CT cases. The local differences in the T. gondii burden observed in our study may be attributed to differences in risky practices during pregnancy, such as eating raw meats or unwashed vegetables, but this remains to be elucidated. Several studies have assessed the spatial pattern of toxoplasmosis. By retrieving local seroprevalence data of T. gondii, Su et al. performed a geospatial analysis in mainland China and Taiwan, showing that the highest infection risk of T. gondii in humans was observed in Taiwan, followed by Xizang and Jilin provinces in mainland China [Reference Su39]. Gotteland et al. applied an agent-based model to investigate the spatial distribution of T. gondii in soil, providing a risk map of infection in Europe [Reference Gotteland40]. To our knowledge, no modelling attempts have been made to explicitly link the spatial pattern of human infection to other intermediate or definitive hosts or the environmental load of T. gondii, which remains to be addressed in future studies [Reference Deng41]. Notably, Japan has a unique system of umbilical cord blood “banks” from which serological data on endemic infections could potentially be obtained [42]. The use of this resource should be considered to determine whether a spatial pattern exists in T. gondii-associated disease burden in order to implement an efficient preventive strategy for CT in Japan.
This study has limitations. First, we assumed that every infected pregnant woman undertaking testing was appropriately diagnosed as infected (or strongly suspected of being infected) and was receiving treatment with spiramycin. Under the Japanese guideline, detection of T. gondii IgG and IgM antibodies in early pregnancy is considered to be sufficient for starting treatment with spiramycin. IgG avidity testing may be used but it is not recommended as a standard practice in the guideline [8]. Considering the possibility of over-diagnosis of acute infection based solely on the results of the detection of specific IgM antibodies, the risk and incidence estimates may be lower than those reported in this study. In a sensitivity analysis to determine the impact of over-diagnosis owing to longer persistence of specific IgM antibodies (which are therefore not markers of acute infection in pregnancy), we found that the estimate of risks and, consequently, numbers of new infections and CT cases in two prefectures were lower, with much uncertainty. T. gondii IgG avidity testing was approved in October 2024 [43]; we can estimate the parameters more robustly in the future when more precise diagnosis of acute infection during pregnancy is available across the country. Second, the serological surveys to which this study refers use various testing methods (three hemagglutination tests [Reference Nishikawa13, Reference Fuse14]; one latex agglutination test [Reference Sakikawa12]; one enzyme-linked immunosorbent assay [16]; three unknown [8, 25]); the most recent survey was conducted 10 years ago [25]. A national-level serological study using a uniform testing method is warranted to provide more reliable and updated information on the susceptible proportion of pregnant people in Japan. Third, except for January 2018 to October 2021 during which the number of pregnancies was mandated to be reported each month, the monthly number of reported live births was leveraged as the number of monthly pregnancies, i.e., we could not include pregnancies that ended with spontaneous or induced abortion. To calculate the exact risk, both risk conditional on delivery and conditional on no delivery are needed, although precisely quantifying pregnancy outcomes along with infection history is challenging. Fourth, in addition to CT being a consequence of primary infection in pregnancy, it can occur in cases of infection reactivation in immunocompromised pregnant people, whereas CT cases in previously immunised women reinfected with a more virulent strain have also been reported [Reference Maldonado44]; our model did not take into account cases of reactivation. Fifth, owing to the limited number of years for which the NDB provides open data, we could not investigate the dynamic trend of incident T. gondii infections and CT cases over a longer time scale. Lastly, but not the least, we assumed only one testing in early pregnancy based on nationwide survey from obstetric facilities in Japan. Motivated by regulatory approval of IgG avidity test last year, testing practice may be changed in future and an alternative model accounting for this revision would be warranted.
In conclusion, as an exercise in putting open health data in Japan to good use, we performed mathematical modelling of T. gondii, estimating the infection risk of T. gondii in pregnancy in Japan. The estimated incidence of infection among pregnant people as well as CT cases, at both national and selected local levels, indicates that toxoplasmosis continues to place a substantial burden on public health in Japan. Given that the magnitude of the burden was comparable to or greater than that of other notifiable congenital infections, far greater attention should be paid to this particular disease, and further studies to elucidate its epidemiology are required.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0950268825100150.
Data availability statement
The epidemiological data analysed in this study and the code to reproduce the results presented in this article are available from https://github.com/koudylan/toxoplasma_japan.
Acknowledgements
The authors thank Analisa Avila, MPH, ELS, from Edanz Group (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author contribution
KN: concept, writing, analysis, collecting data. HN: planning, concept, writing, analysis, guarantor.
Funding statement
H.N. received funding from Health and Labour Sciences Research Grants (grant numbers 20CA2024, 21HB1002, 21HA2016, and 23HA2005), the Japan Agency for Medical Research and Development (grant numbers JP23fk0108612 and JP23fk0108685), JSPS KAKENHI (grant numbers 21H03198 and 22 K19670), the Environment Research and Technology Development Fund (grant number JPMEERF20S11804) of the Environmental Restoration and Conservation Agency of Japan, the Daikin GAP Fund of Kyoto University, Japan Science and Technology Agency SICORP program (grant numbers JPMJSC20U3 and JPMJSC2105), the CREST program (grant number JPMJCR24Q3), and RISTEX program for Science, Technology, and Innovation Policy (grant number JPMJRS22B4). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests
K. N. is employed at Janssen Pharmaceutical K.K. H. Nishiura has no competing interest to declare.







